AIS LIMS Modules
AIS LIMS is a generic Laboratory Information Management System that provides the tools required to help operate and efficiently manage your laboratory processes. The AIS LIMS system can be configured to meet virtually any organisation’s specific needs and requirements, allowing you to save time and costs by automating tasks, integrating equipment and systems and streamlining processes.
Security
Protecting your AIS LIMS software is important to us. Our Laboratory Information Management System software is protected by 64-bit encrypted password security which ensures only designated and trusted users can access your sample data. Should you wish to have different security levels and accessibility rights; users can be given profiles which restrict them to certain areas. Users will normally log themselves out of the system, but the system can also be configured so that it automatically logs users out after a specified period of inactivity.
Navigation
AIS LIMS is accessed via a simple series of user-configured menus and toolbars controlled using various security levels or user profiles. Navigation can also be achieved via our “Control Centre” hub, showing views and actions relevant to a wide array of user activities. User profiles can be assigned, allowing management of access to restricted activities such as sample and results approval for example.
Sample Registration
AIS LIMS allows samples to be registered quickly and efficiently into the system. All samples logged into your Laboratory Information Management System will be given unique sample numbers. All actions performed against a sample are recorded to ensure traceability and regulatory compliance. Samples can be registered individually or in groups and can be registered from files created from sampling programs, stability profiles, or other systems such as AIS labPortal.
The screens used for registration are highly configurable, allowing you to record the information that is relevant to your operation and in familiar terminology. Labels displaying sample numbers as barcodes, plus relevant and useful sample information can be designed and produced, as can complete lists of registered samples and required tests. Sample receipts/confirmations containing registered sample details and pricing can be emailed directly to clients for confirmation before commencing analysis.
Sample Tracking
Regulatory compliance requires the need for sample traceability throughout the laboratory process. AIS Laboratory Information Management System provides this functionality, allowing you to barcode sample containers/aliquots and track their locations throughout the process. This ensures the lab and your clients are always aware of where samples are during the lab life cycle.
Scheduling
AIS LIMS offers several ways in which work can be scheduled and automated. Worksheets can be generated to be used as result entry forms and worklists can be generated for daily laboratory workloads, instrument loading etc. These are simple to use and allow the inexperienced user to enter data into the system quickly and accurately, limiting the opportunity for errors.
Results Entry
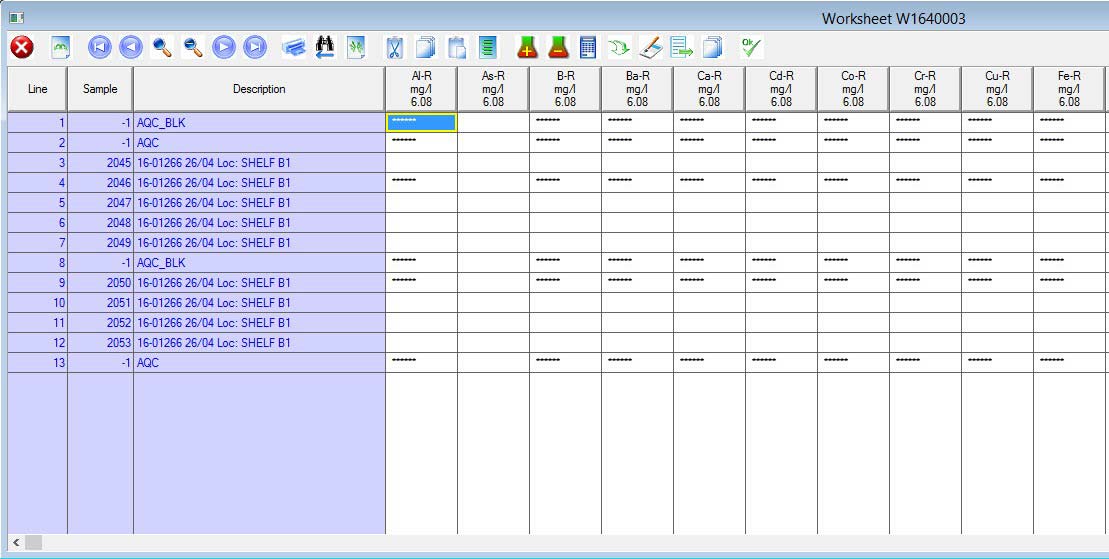
AIS Laboratory Information Management System allows results to be entered in a variety of formats supporting all required input. It allows results to be entered manually at the keyboard or automatically from instrument files and for simpler interfaces (e.g. balances) through a keyboard capture system. Data captured directly from laboratory instruments gives benefits in data accuracy and in the speed of data entry, which improves the performance and efficiency of the lab. User training records, instrument records and analytical quality control (AQC) targets are utilised by the software for results entered or generated in order to ensure validity, accuracy and precision.
AIS LIMS supports the use of user-defined calculations in its worksheets and has the ability to automatically extract results from the database and process calculations internally. This automation removes errors made during manual calculation processing and eliminates the need for costly retesting. Any changes to the input values automatically trigger recalculation of derived values.
Validation and Approval
The sample approval process allows designated users with permissions to allocate “validated”, "approved", "failed", or “withdrawn” status to a sample or group of samples. During the approval stage, (which may also be automated) the user can examine all data associated with the sample. This includes associated documents, audit trail, quality (AQC) data and historical results so that these can be evaluated before approving samples. General, specific, or statistical limits which are applicable can be displayed and limit infringements highlighted and brought to the attention of the user before approving results.
Reporting
AIS LIMS includes a proprietary report designer which allows trained users to create any number of report templates. AIS LIMS also supports third-party reporting tools such as Microsoft Excel. This flexibility in 'output source' ensures compatibility and customer satisfaction.
AIS LIMS reporting modules allow you to deliver results to your customers in a number of different formats. We offer several different report styles for both hard copy and electronic output of both individual and multi-sample reports which the user is able to select and customise.
Views
AIS LIMS supports any number of user-defined views of LIMS data as tables or graphs from which many actions can be launched, using SQL queries. Views may be associated with user profiles so relevant views are shown upon logging into LIMS. View contents can be automatically updated on a timed basis or on completing certain actions.
Analytical Quality Control
Analytical Quality Control (AQC) is a framework for monitoring the accuracy and precision of analytical measurements. The main purpose of AQC is to:
- Provide confidence in analytical measurements
- Demonstrate compliance with performance targets
- Obtain timely warnings of deteriorating trends
- Validate new analytical methods or instrumentation
- Meet requirements of accreditation schemes (e.g. UKAS, MCERTS, INAB)
AIS Laboratory Information Management System AQC software is an optional accessory which can be purchased alongside the LIMS to provide a basis for interactively scheduling, recording and checking analytical results against known quality standards. This ensures the reliability of results and helps to improve performance and maintain accuracy.
The AQC module is capable of measuring multiple standards and known reference materials as well as duplicate sample analyses and recovery in spiked samples. AQC warnings and actions are audited and are displayed during result entry and approval processes. AQC results can be reported as a statistical summary and control charts can be generated to allow trends to be monitored. Automated processes can be configured to generate a series of AQC charts for selected determinations. AQC breaches are automatically logged and stored as part of the software’s audit trail which is essential for traceability.
For further information about AIS Laboratory Information Management System Software solutions please call +44 (0) 1531 828930 or alternatively email marketing@ais-lims.com